August 2022
Intermediate result from partner: ABEE
Electrochemical model involves solving of complex partial differential equations that can be used to describe the physical processess such as, diffusion, migration and reaction kinetics inside the batteries. The electrochemical model is coupled with a mechanical model and is used to analyze the ionic conductivity and mechanical intercation between electrodes and electrolyte.
- Objective: The main objective is to develop a model that can provide insights into complex interfaces formed between electrodes and electrolytes and study the solid electrolyte’s transport properties.
- Research: State-of-the-art continuum P2D models were adapted to model lithium-metal solid-state electrolyte cells. Within the modeling framework, the conductivity of solid-electrolyte and mechanical interactions were included.
- Result: With the developed model, hybrid electrolytes were investigated. POEO/LLZO electrolyte was developed by CICe (at 90°C) and for PEO/LLZO electrolyte was developed by SCHOTT (at 60°C). Conductivity data from CICe shows strongly decreasing effective conductivity by increasing the LLZO fraction. However, the conductivity data from SCHOTT shows increasing conductivity with increasing LLZO content. The electrochemical model coupled with the mechanical model showed that during the discharge, there is an initial change of volume due to the concentration gradient that stabilizes after two minutes (see figure 1).
- What will it be used for: The results obtained can provide insights on the ionic conductivity of the electrolyte that can be useful in achieving the targeted power and energy density, as well as provide understanding on the stress generated as a consequence of the volume change in the lithiation and delithiation phases.
- Impact: The results can be used to improve the development of electrolyte and helps in understanding of impact of materials on electrolyte’s properties.
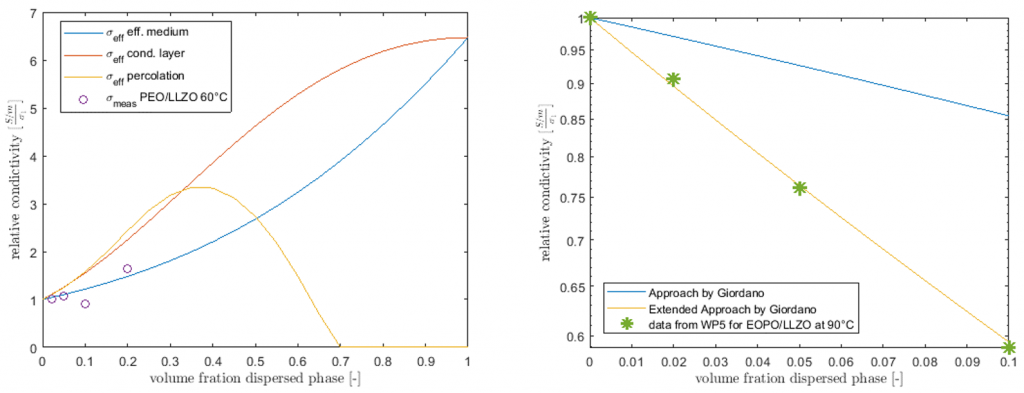
Figure 1: Conductivity modeling for POEO/LLZO electrolyte from CICe (90°C) (right) and for PEO/LLZO electrolyte from SCHOTT (60°C) (left).
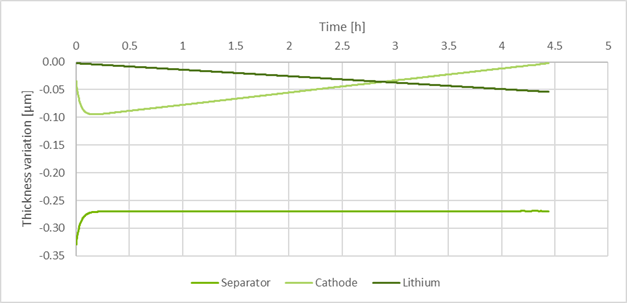
Figure 2: Variation of the thickness of each of the components during a single discharge at C/5 of a cell subjected to 1 MPa external pressure.